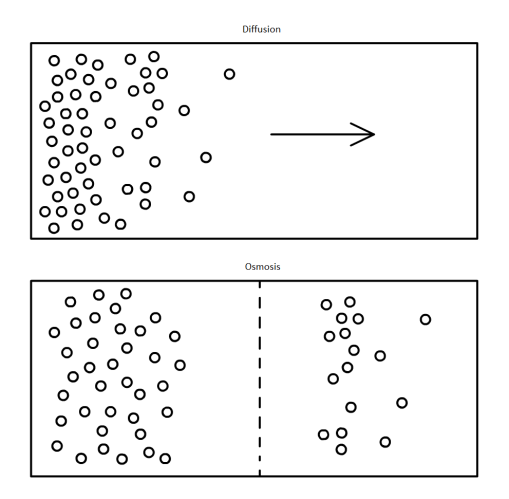
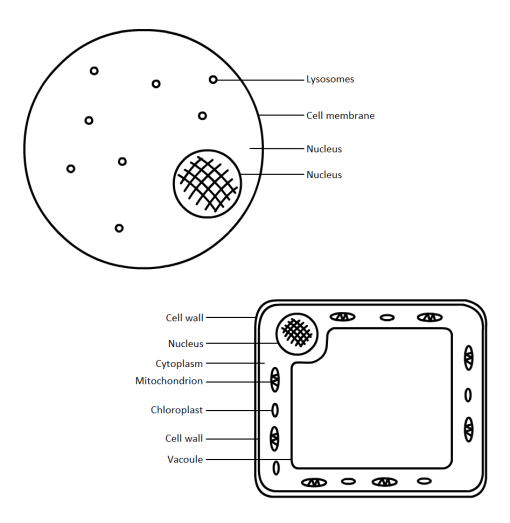
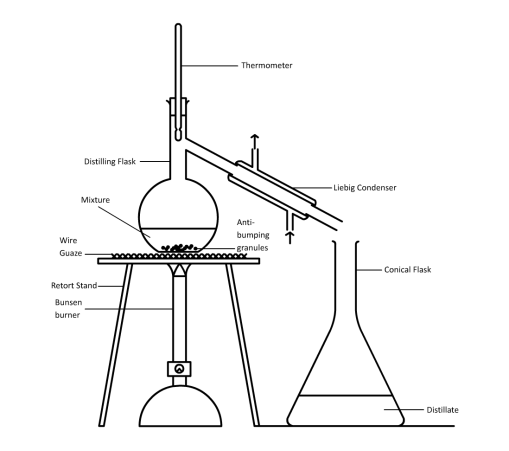
Last topic… Photosynthesis.
Here’s a bit of history:
In the mid-17th Century, biologist Jan Baptist van Helmat suspected that plant growth had tocome from water, after test he conducted showed no change in soil amount. However, carbon rich organic molecules can be found in plants. Water does not provide carbon. Thus, from that point, scientists discovered that water was an important element for photosynthesis, but not the sole component. The process of making food was thus defined as photosynthesis.
Photosynthesis is a process which
- Carbon Dioxide, water and light is trapped by chlorophyll to make food in the form of Glucose, with Oxygen given off in the process.
- The chemical process of Photosynthesis is: 6 Co2 + 6 O2 — light — C6h1206 + 02.
Now let’s look into chloroplasts…
- Chloroplasts are disc like structures found in plant cells.
- Rows of chlorophyll are stacked up in the chloroplasts.
- Leaves are green due to the chlorophyll reflecting the green pigment of light.
- Thus, plants have near-zero growth when exposed to green light.
Now, glucose…
- Glucose is the basic building block of the plant. They are stored as starch when in excess.
- Potato and yams are examples of storage parts of plants.
Finally, stomata…
- Stomata are small openings in the structure of the leaves that allow gaseous exchange with the air.
- Flanked by 2 guard cells, each stomata open and close at regular intervals.
- These stomata are usually found on the underside of the leaves, as the underside is not exposed to as much sunlight as the topside, thus preventing water loss through evaporation.
Now, for a few tests…
Carbon Dioxide test:
- C02 bubbled through Calcium Hydroxide, a white precipitate will be formed in the Calcium Hydroxide.
O2 test
- O2 will relight a glowing splint
- Brighter glow is also counted as a presence of oxygen
Starch test
- A variegated leaf is destarched for 4 days, before boiled and soaked in ethanol to kill it and decolourise it.
- Next, Iodine solution is dropped onto the leaf.
- If the leaf turns brown, Starch is absent.
- If it turns blue, Starch is present